The Rapid COVID-19 Antigen Test is a colloidal gold immunochromatography intended for the qualitative detection of nucleocapsid antigens from COVID-19 in human nasal swabs ,throat swabs or saliva from individuals who are suspected of COVID-19 by their healthcare provider. The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. Results are for the identification of COVID-19 nucleocapsid antigen. The antigen is generally detectable in upper respiratory samples or lower respiratory samples during the acute phase of infection. The positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. The positive results do not rule out bacterial infection or co-infection with other viruses. The antigen detected may not be the definite cause of disease. The negative results do not rule out COVID-19 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. The negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19 and confirmed with a moecular assay, if necessary for patient management.
This reagent is based on colloidal gold immunochromatography assay. During the test, specimen extracts are applied to the Test Cards. If there are COVID-19 antigen in the extract, the antigen will bind to the COVID-19 monoclonal antibody. During lateral flow, the complex will move along the nitrocellulose membrane toward the end of the absorbent paper. When passing the test line (line T, coated with another COVID-19 monoclonal antibody) the complex is captured by COVID-19 antibody on test line shows a red line; when passing the line C, colloidal gold-labeled goat anti-rabbit IgG is captured by control line (line C, coated with rabbit IgG) shows a red line.
The following components are included in the Rapid COVID-19 Antigen test kit.
Materials Provided:
|
Sample Type |
Materials |
|
Saliva(only) |
|
Materials Required but not Provided:
1. Timer
2. Tube rack for specimens
1. Store the product at 2-30℃, the shelf life is 24 months tentatively.
2. Test cassette should be used right after opening the pouch.
3. Reagents and devices must be at room temperature(15-30℃) when used for testing.
Throat Swab Specimen Collection:
Let the patient's head tilt slightly, mouth open, and make "ah" sounds, exposing the pharyngeal tonsils on both sides. Hold the swab and wipe the pharyngeal tonsils on both sides of the patient with moderate force back and forth for at least 3 time.
Saliva Specimen Collection by Swab:
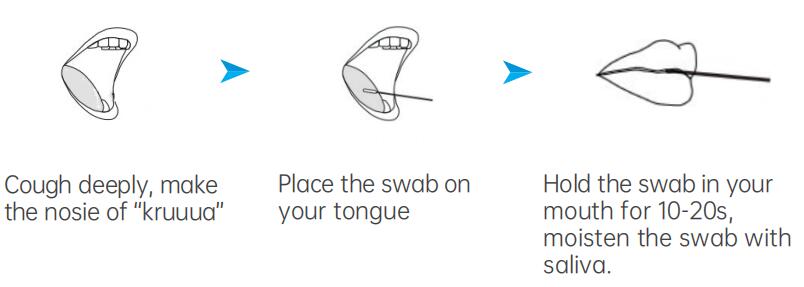
Saliva Specimen Collection by Saliva Collection Device:
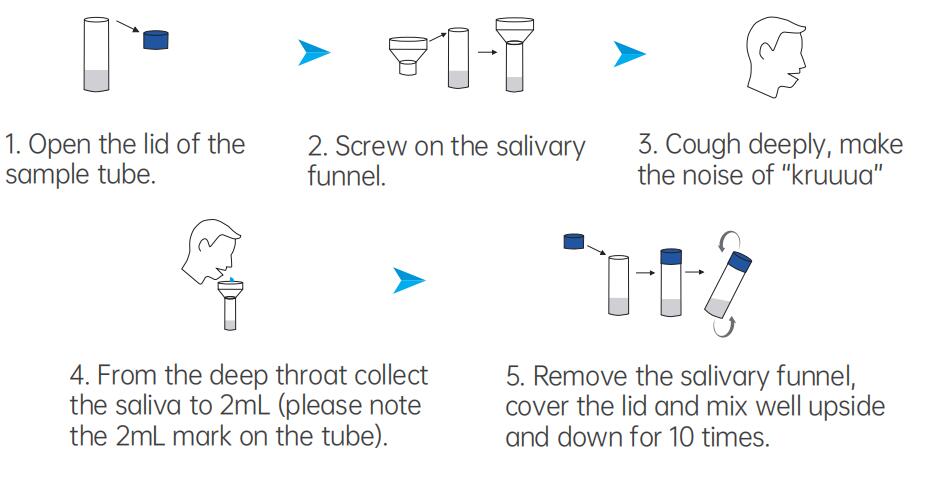
Specimen Transport and Storage:
Samples should be tested as soon as possible after collection. Swabs or saliva specimen can be stored in Extraction Solution for up to 24 hours at room temperature or 2° to 8°C. Do not freeze.
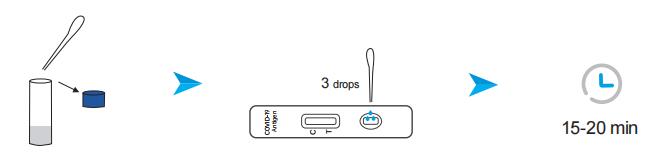
1. The test should be operated at room temperature (15-30°C).
2. Add the specimens.
Saliva Specimen (from Saliva Collection Device):
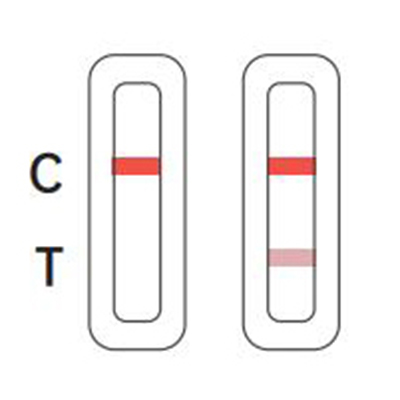
Positive
There is coloration on line C, and a colored line appeared T line which is lighter than C line, or there
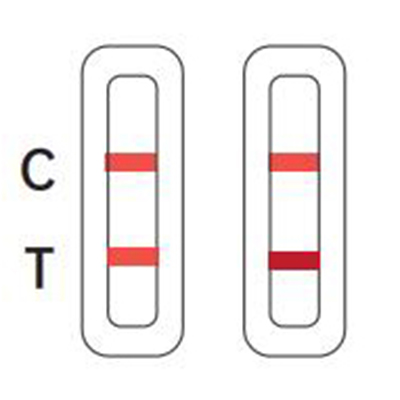
Negative
There is coloration on line C, and a colored line appeared T line which is darker or equal than
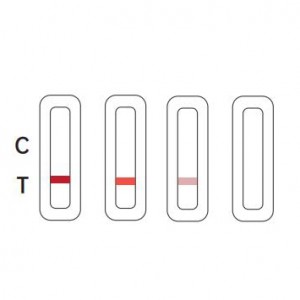
Invalid
There is no coloration on line C, as shown in the following pictures. The test is invalid or an error
Negative(-): Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results would be confifirmed by a molecular testing method, if necessary, for patient management Control.
Positive(+): Positive for the presence of SARS-CoV-2 antigen. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or coinfection with other viruses. The antigen detected may not be the defifinite cause of disease.
1.Clinical performance was evaluated with frozen samples, and test performance may be different with fresh samples.
2.Users should test specimens as quickly as possible after specimen collection.
3.Positive test results do not rule out co-infections with other pathogens.
4.Results of COVID-19 antigen test should be correlated with the clinical history, epidemiological data, and other data available to the clinician evaluating the patient.
5.A false-negative test result may occur if the level of viral antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly; therefore, a negative test result does not eliminate the possibility of COVID-19 infection.
6.The amount of antigen in a sample may decrease as the duration of illness increases. Specimens collected after day 5 of illness are more likely to be negative compared to a RT-PCR assay.
7.Failure to follow the test procedure may adversely affect test performance and/or invalidate the test result.
8.The contents of this kit are to be used for the qualitative detection of COVID-19 antigens from saliva specimens only.
9.The reagent can detect both viable and non-viable COVID-19 antigen.The detection performance depends on antigen load and may not correlate with other diagnostic methods performed on the same specimen.
10. Negative test results are not intended to rule in other non- COVID-19 viral or bacterial infections.
11. Positive and negative predictive values are highly dependent on prevalence rates. Positive test results are more likely to represent false positive results during periods of little/no COVID-19 activity when disease prevalence is low. False negative test results are more likely when prevalence of disease caused by COVID-19 is high.
12. This device has been evaluated for use with human specimen material only.
13. Monoclonal antibodies may fail to detect, or detect with less sensitivity, COVID-19 viruses that have undergone minor amino acid changes in the target epitope region.
14. The performance of this test has not been evaluated for use in patients without signs and symptoms of respiratory infection and performance may differ in asymptomatic individuals.
15. The kit was validated with the assorted swabs. Use of alternative swabs may result in false negative results.
16. Users should test specimens as quickly as possible after specimen collection.
17. The validity of Rapid COVID-19 Antigen Test has not been proven for dentifification/confifirmation of tissue culture isolates and should not be used in this capacity.

